We guarantee the highest quality and validation of the system
History of the company
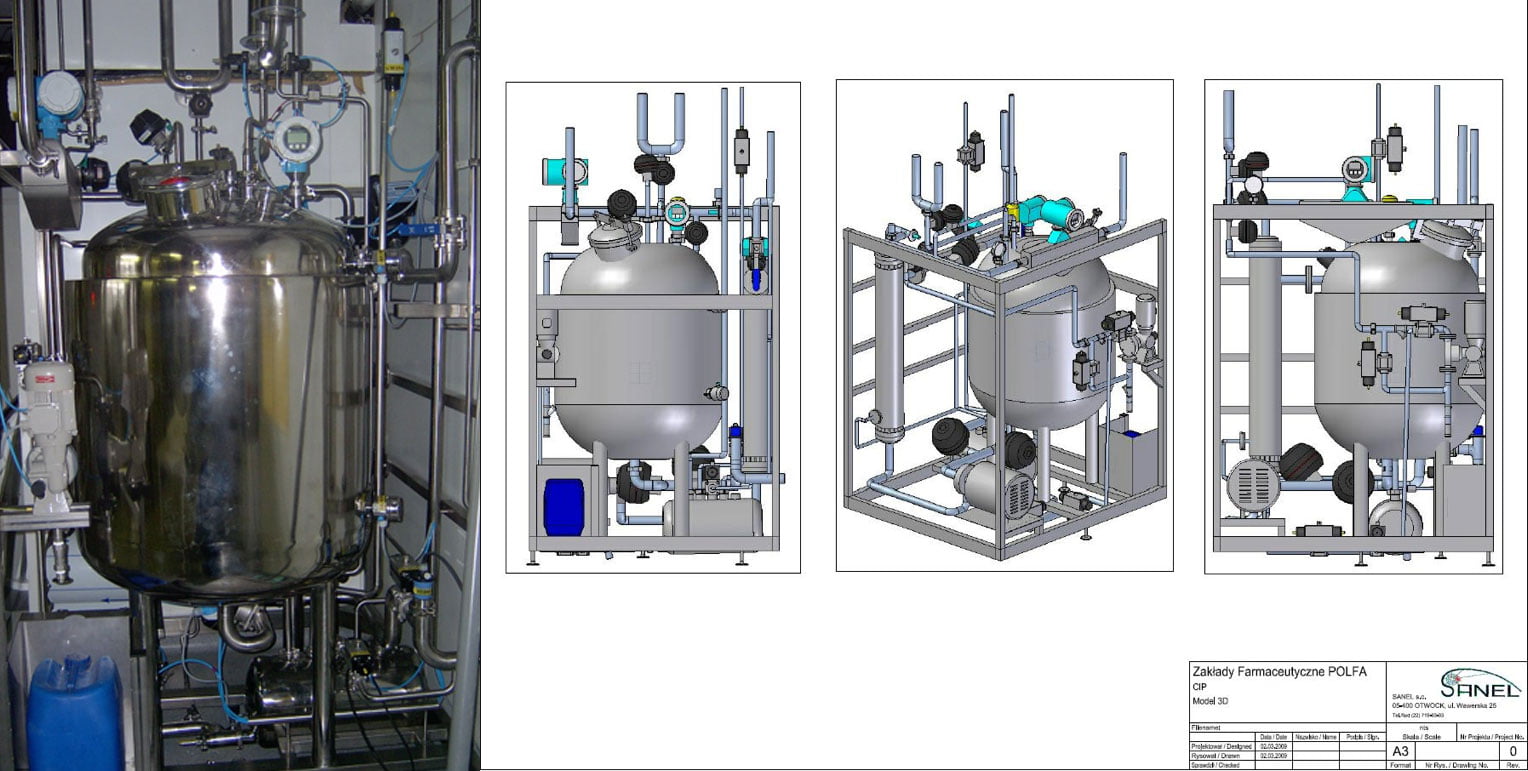
SANEL spółka jawna was established on 01/03/1989 and from its beginning up to the present day it has been a privately-owned company. The company deals with the production of complete technological systems for the pharmaceutical and cosmetic industries in Poland and other parts of the world. It is constantly developing and offers services and products meeting high hygienic requirements. The dynamic growth of the company and the new markets have resulted in the opening of a representative office in Kiev, Ukraine, and an independent company, Sanel.Kz, in Kazakhstan. Our company implements comprehensive installations, including the design and delivery of materials and equipment and their servicing.

Piotr Zawistowski – Owner of Sanel

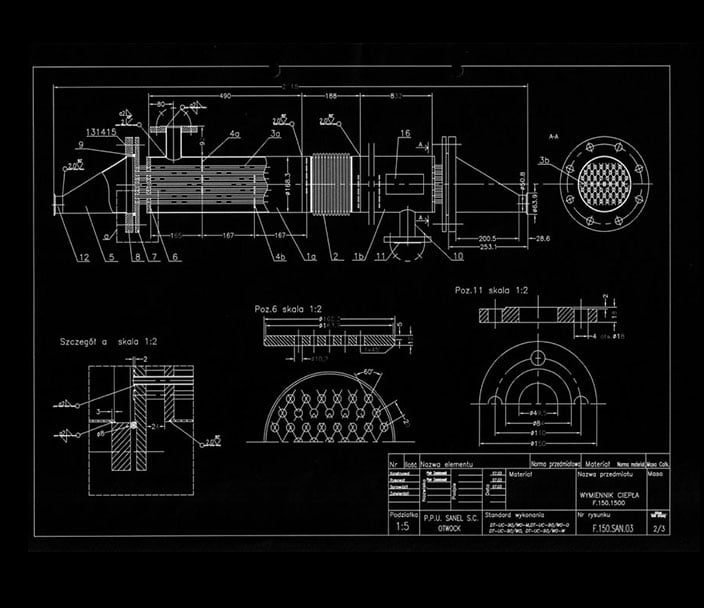
Our company has extensive experience in making high alloy steel installations for the pharmaceutical and cosmetic industries. We possess inverters for orbital TIG welding with the ability to document the welding process, with heads for welding pipes with a diameter of 6 to 160 mm, a video camera with the possibility of photographic documentation and video recordings of welds made, up to the probe length. We guarantee assembly, welding and inspection according to procedures that guarantee the highest quality and validation of the installation.
Area of operations

SANEL offers:
Quality Control
We implement our installations in accordance with the following requirements:
cGMP, CFR11, GAMP5, Baseline Pharmaceutical Engineering Guide “Water and Steam Systems” Volume 4.
PLC system
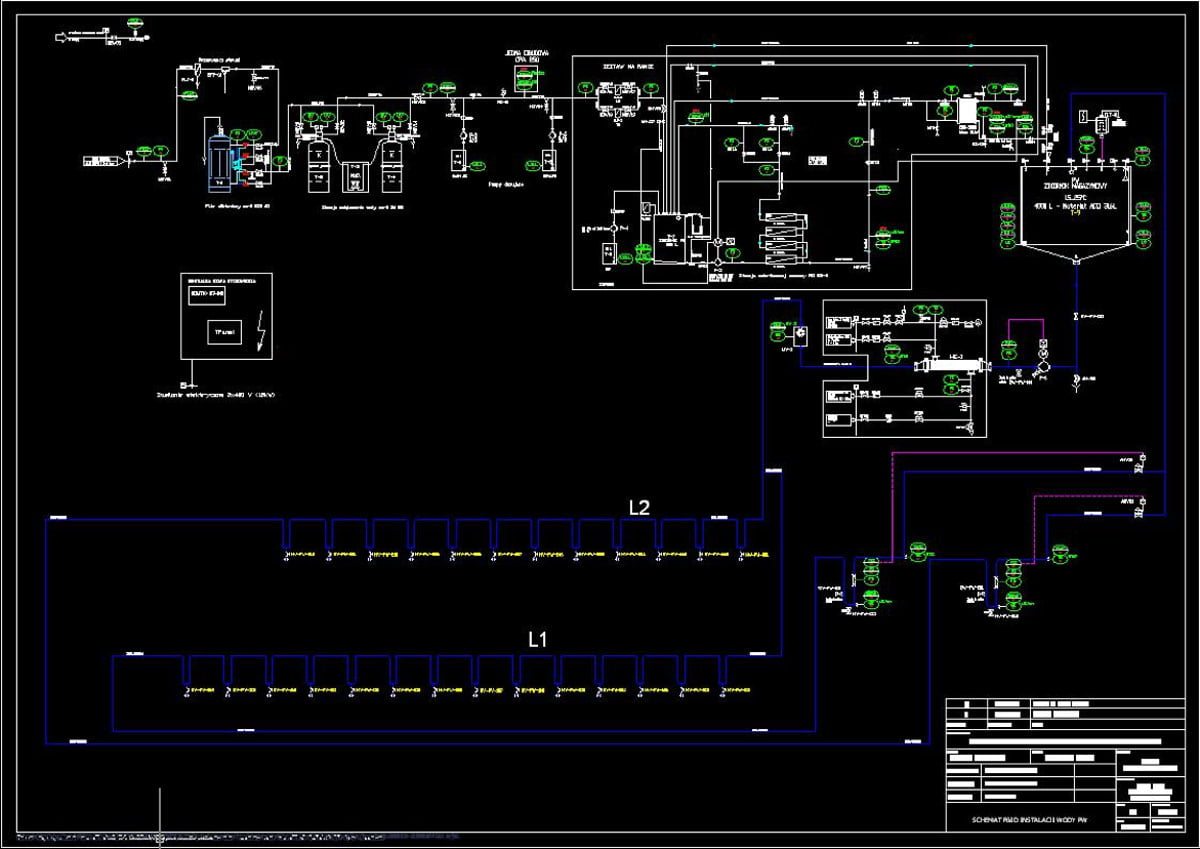
P&ID
HMI screens
Our partners:
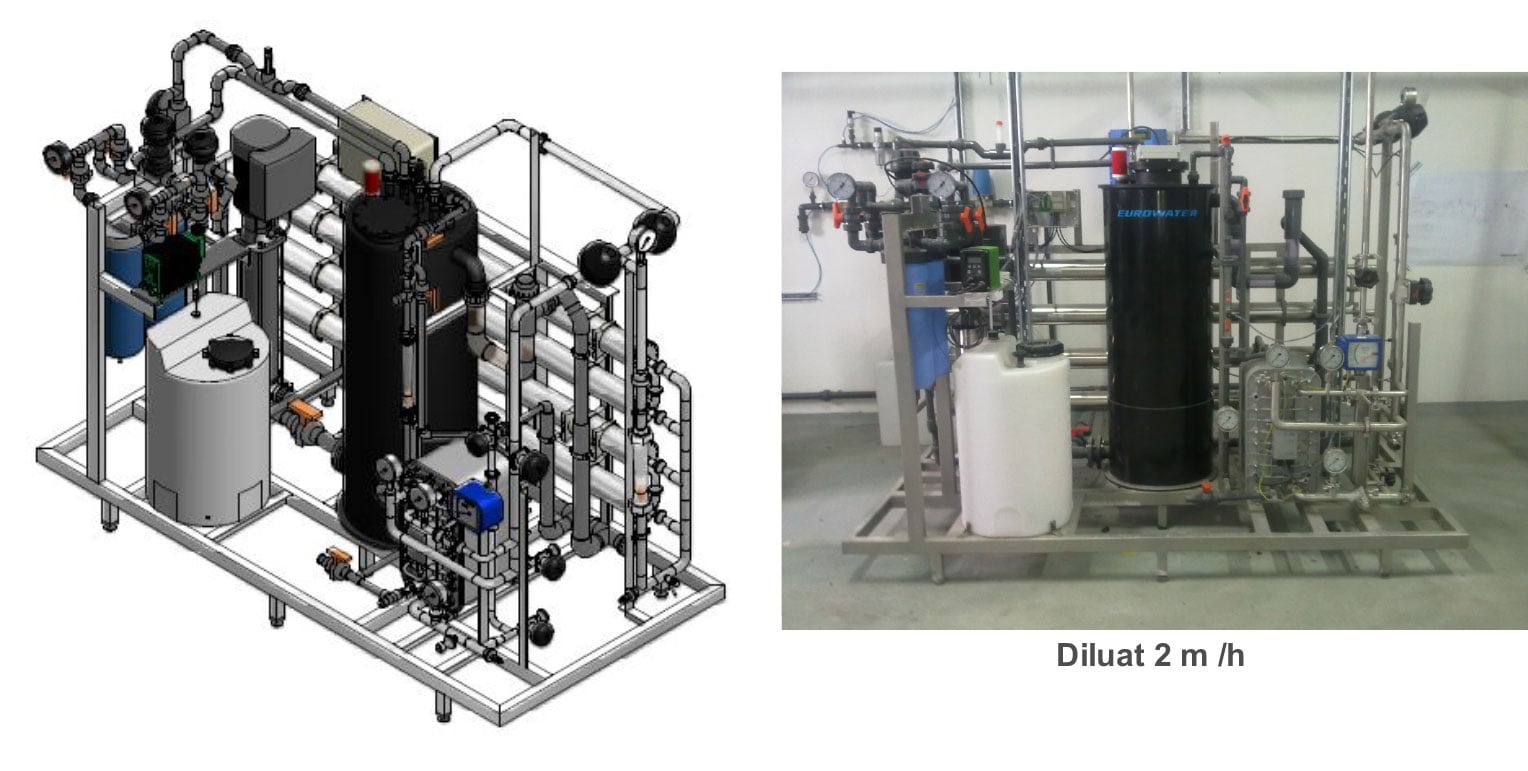
We use proven components in our solutions from such companies as: SIEMENS, BECKHOFF, SCHRACK, LG, INDUSOFT, ENDRESS&HAUSER, BURKERT, GEMU, DOCKWEILER, EUROWATER and GEA, which allows us to easily adapt to the requirements and capabilities of any investor.